Amazing experience with Epiphyllum oxipetalum
 Aug 15, 2020 • 10:13 AM UTC
Aug 15, 2020 • 10:13 AM UTC Unknown Location
Unknown Location 140x Magnification
140x Magnification Microorganisms
Microorganisms
TCIS Outreach
We are a group of students, volunteers and staff working with TIFR Hyderabad's Science Education and Outreach program: http://www.tifrh.res.in/~outreach/
39posts
26comments
2locations

Epiphyllum oxypetalum is often described as ‘Queen of the night cactus’. In India it is considered a sacred plant and called ‘ Brahma Kamalam ’, though there is much controversy about whether or not it is the ‘true’ Brahma Kamalam. In any case there is a lot of mystique associated with this plant. It blooms sometime between July to October, in a characteristic style: as the bud matures its stalk bends and turns the flower upwards, like an orchid. It blooms in the night with mild, pleasant odour.
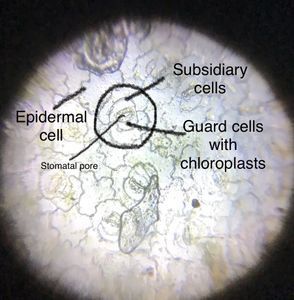
Six months ago I planted one phylloclade, a flat leaf-life stem which grows into a plant. Back in May, I posted on Microcosmos some pictures of this plant and its stomata.
Six months ago I planted one phylloclade, a flat leaf-life stem which grows into a plant. Back in May, I posted on Microcosmos some pictures of this plant and its stomata.

Recently my family and I got really excited to find a bud on the plant!

We watched over a few days as the stalk began to twist upwards.

After five days of our first noticing the bud, at 8 pm one evening, the flower finally started to bloom!

The flower took almost one hour to bloom completely.

Watch its beauty!

I felt blessed, as would any Indian watching the blooming Brahma Kamalam. It’s an amazing experience! People commonly do pooja with this flower. But me, it came into my mind to observe its pollen under the Foldscope. On a strip of cellotape I collected pollen from the flower.
My curiosity was raised to observe pollen germination. I have a solution that Chandrika gave me a month ago to observe pollen germination (see postscript).
My curiosity was raised to observe pollen germination. I have a solution that Chandrika gave me a month ago to observe pollen germination (see postscript).
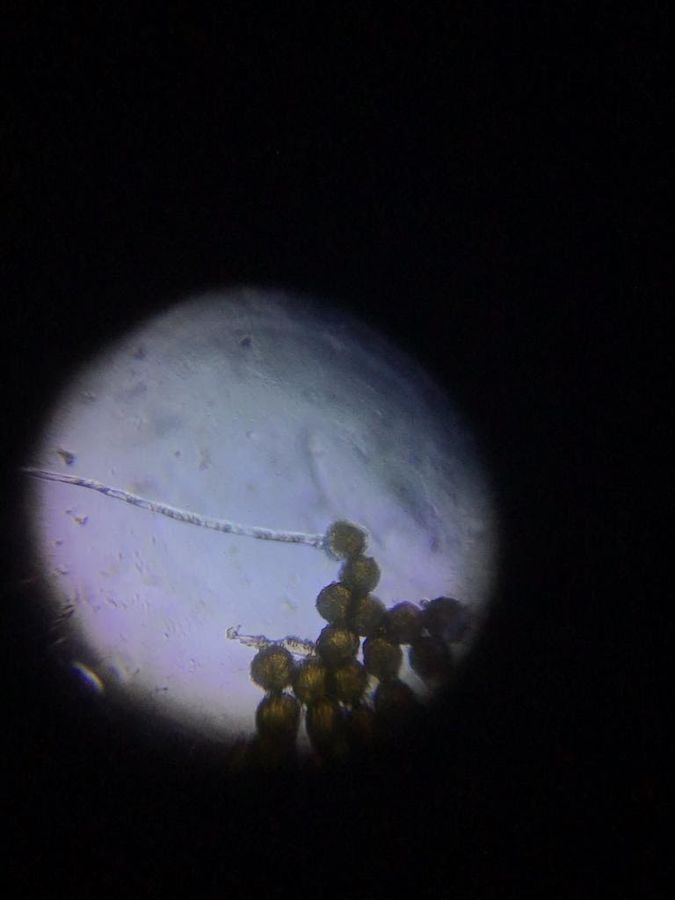
I tried the solution with pollen from Caesalpinia pulcherrima. Only one or two germinated. I was not even sure whether this was a pollen tube. I tried the solution with pollen from Hibiscus, from the garden and also from my potted plant. No interesting results. I lost interest and kept the solution back in the refrigerator.

It was 11:30 pm the night that the Brahma Kamalam bloomed, and I put its pollen in a drop of the magic solution.
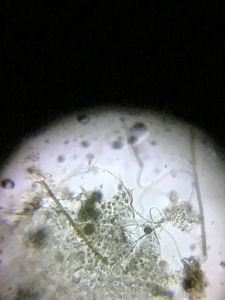
Within ten minutes I saw some of the pollen grains had lost their circular shape and at one point bulked out. I concentrated on just one pollen grain and suddenly, the bulged part broke and an unbelievably looooooong, continuous tube rapidly streamed out of that pollen grain. Just watch this real time video! Surely this cannot be a pollen tube?
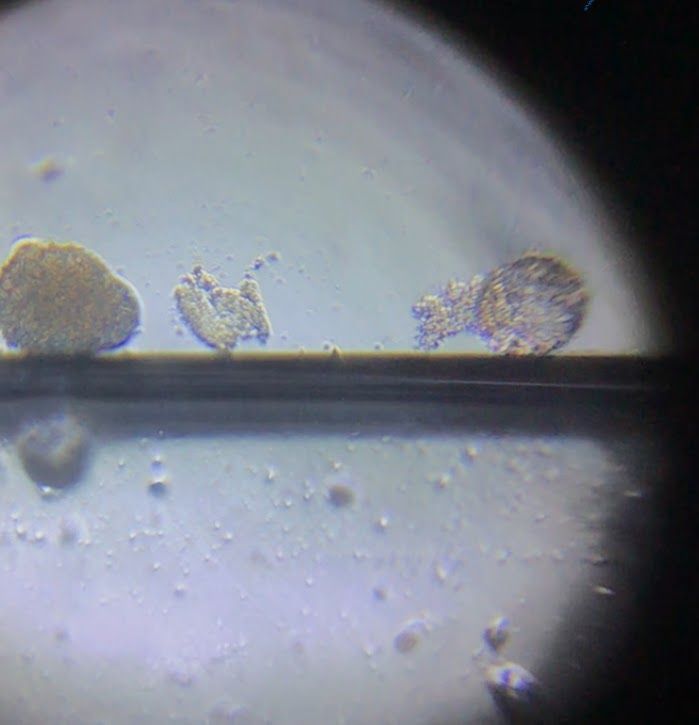
I then found on this slide one other pollen grain that had bulged out and another pollen grain broken up.

In the same slide, I observed one pollen grain with three, four, and more “pollen tubes”!
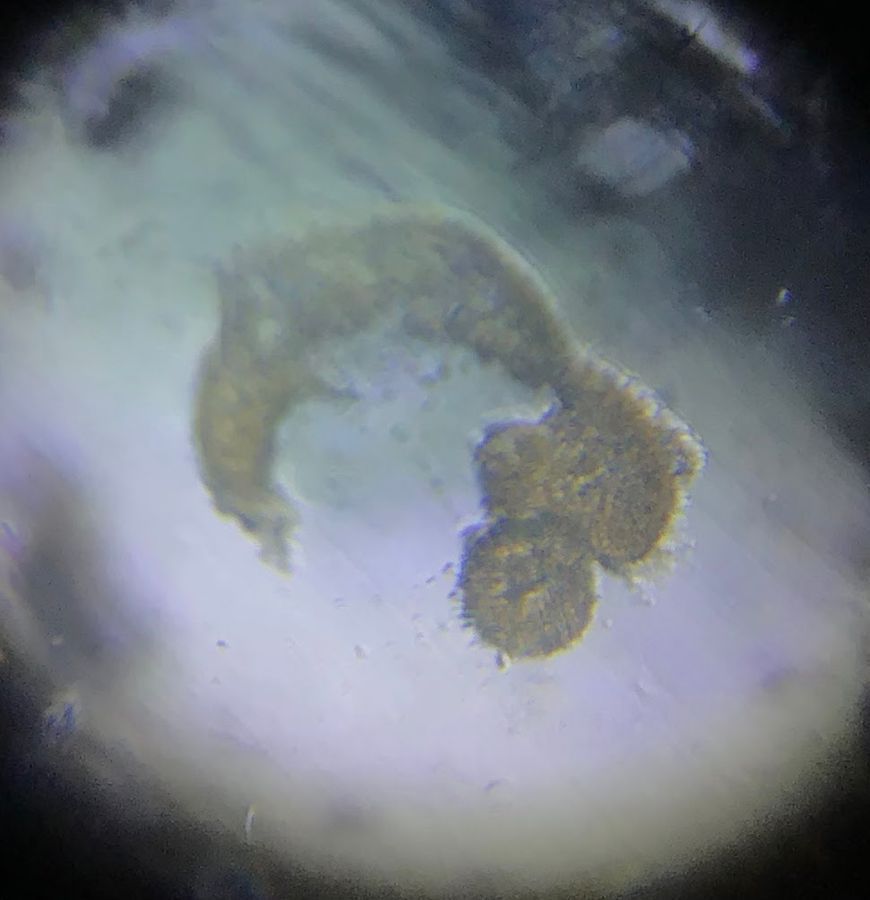
From one pollen grain protoplasm seemed to have extruded out. Is it because of exosmosis?
It was already 1:10 am. I stopped observing. The next morning the flower had wilted but I collected another sample of pollen from it. By the time I started my observations, it was noon. The next set of observations are all from one slide.
It was already 1:10 am. I stopped observing. The next morning the flower had wilted but I collected another sample of pollen from it. By the time I started my observations, it was noon. The next set of observations are all from one slide.

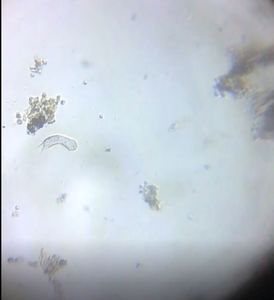
As I was focusing, I saw another very long “pollen tube” had already emerged out.
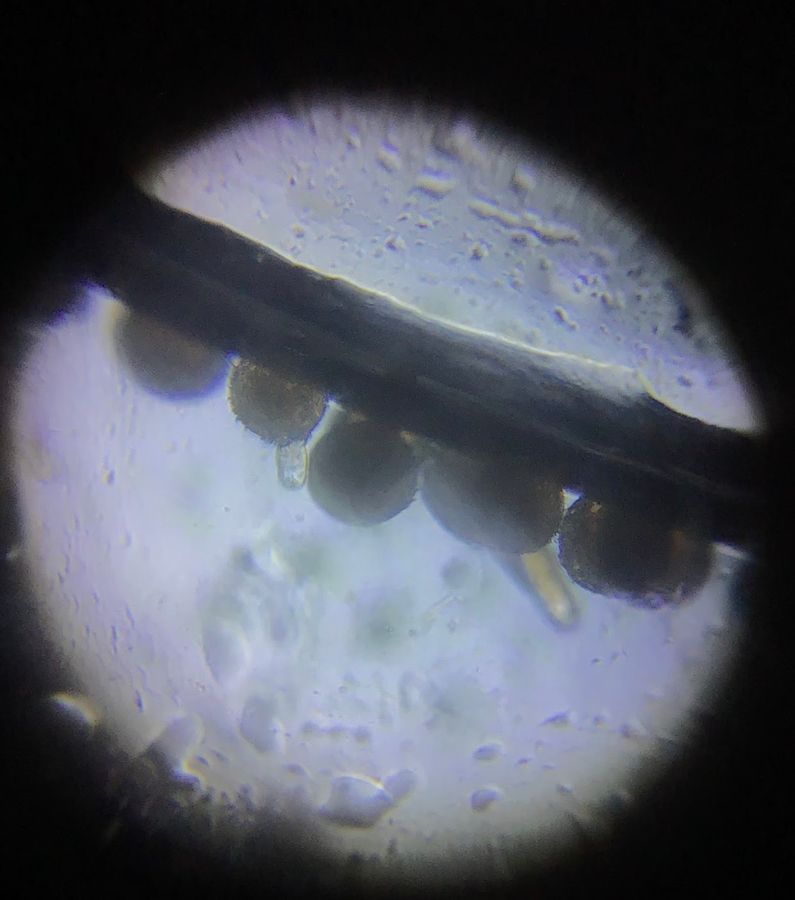
Then I saw a couple of tubes that were emerging slower. These may be real pollen tubes perhaps.
Another of the very long emerging tubes and several similar ones floating in the solution.
All the above observations were from one slide.
Later, when I focused this slide with the cellophane I saw that the pollen grains were squeezed into almost doughnut-like shapes. This must be plasmolysis, caused by shrinking or loss of plasma.
At the end of my observations, the delicate flower had wilted completely. To continue these observations I must wait for another flower to bloom. In the meantime, I will learn how to use time-lapse while observing.
I am grateful to the Foldscope team for creating such a wonderful instrument to help anyone observe these beautiful phenomena anywhere, anytime.
Cheers,
Ashalatha
Postscript
Ashalatha shared an educational video by Amrita Center for Research in Analytics, Technologies & Education ( AmritaCREATE ) on pollen germination. We prepared the ‘germinator solution’ by taking 100 ml distilled water and dissolving 20 mg potassium nitrate (KNO 3 ) + 30 mg magnesium sulphate (MgSO 4 ) + 10 mg boric acid (H 3 BO 3 ) + 10 g sucrose (C 12 H 22 O 11 ). Our advanced research labs did not have potassium nitrate, so we went ahead and synthesised it. Turns out potassium nitrate has wide applications, all the way from fertilizers to firecrackers and gunpowder! Praveen Taware (a graduate student at TIFRH) titrated potassium hydroxide (KOH) with nitric acid (HNO 3 ). A simple neutralization (exothermic) reaction occurs in which potassium nitrate (KNO 3 ) precipitates. We then dried and weighed the powder. Caution: Please do not perform this experiment without a responsible adult or chemist around. We thank Abhinanda Kundu (postdoctoral fellow, TIFRH) and Shamasree Ghosh (graduate student, TIFRH) for support in procuring the chemical compounds.
During a discussion, @Laksiyer suggested that the chemicals could be replaced by unburst crackers (potassium nitrate), epsom salt (magnesium sulphate), carrom board powder (boric acid) and sugar (sucrose). Of course then the proportions would call for a quite bit of a guesswork!
— Chandrika
Later, when I focused this slide with the cellophane I saw that the pollen grains were squeezed into almost doughnut-like shapes. This must be plasmolysis, caused by shrinking or loss of plasma.
At the end of my observations, the delicate flower had wilted completely. To continue these observations I must wait for another flower to bloom. In the meantime, I will learn how to use time-lapse while observing.
I am grateful to the Foldscope team for creating such a wonderful instrument to help anyone observe these beautiful phenomena anywhere, anytime.
Cheers,
Ashalatha
Postscript
Ashalatha shared an educational video by Amrita Center for Research in Analytics, Technologies & Education ( AmritaCREATE ) on pollen germination. We prepared the ‘germinator solution’ by taking 100 ml distilled water and dissolving 20 mg potassium nitrate (KNO 3 ) + 30 mg magnesium sulphate (MgSO 4 ) + 10 mg boric acid (H 3 BO 3 ) + 10 g sucrose (C 12 H 22 O 11 ). Our advanced research labs did not have potassium nitrate, so we went ahead and synthesised it. Turns out potassium nitrate has wide applications, all the way from fertilizers to firecrackers and gunpowder! Praveen Taware (a graduate student at TIFRH) titrated potassium hydroxide (KOH) with nitric acid (HNO 3 ). A simple neutralization (exothermic) reaction occurs in which potassium nitrate (KNO 3 ) precipitates. We then dried and weighed the powder. Caution: Please do not perform this experiment without a responsible adult or chemist around. We thank Abhinanda Kundu (postdoctoral fellow, TIFRH) and Shamasree Ghosh (graduate student, TIFRH) for support in procuring the chemical compounds.
During a discussion, @Laksiyer suggested that the chemicals could be replaced by unburst crackers (potassium nitrate), epsom salt (magnesium sulphate), carrom board powder (boric acid) and sugar (sucrose). Of course then the proportions would call for a quite bit of a guesswork!
— Chandrika
Sign in to commentNobody has commented yet... Share your thoughts with the author and start the discussion!

 0 Applause
0 Applause 0 Comments
0 Comments