Building a micro-aquarium using the ditch-spacer slide technique
 Jun 14, 2016 • 8:49 PM UTC
Jun 14, 2016 • 8:49 PM UTC Unknown Location
Unknown Location 140x Magnification
140x Magnification Microorganisms
Microorganisms
Laks Iyer
Human observer of life. https://sukshmadarshin.wordpress.com
97posts
1255comments
5locations

View in Media Gallery
One of my long-term aspirations is to possess a micro-aquarium, where microscopic life is constrained in a small slide volume that I could watch for days and see aspects of the cycles of the various life forms. How cool would that be? Yet, it all only seemed like a fancy… until this May, where at the National Geographic Bioblitz, I had the wonderful opportunity to meet Alice Dai, Jim Cybulski, Matt Bull (from the Prakash Lab) and super-user Matt Rossi. Alice showed us this wonderful PVC material that she was playing with, where she could punch in a hole and fill it with liquid sample and hermetically seal it with PVC at both ends. Around the same time Manu showed how he solved the fixed focus problem and also demonstrated dark field with a table lamp (see https://microcosmos.foldscope.com/?p=16093 ). At that very moment, I knew that a life-long dream was very close to being fulfilled. So here is a preliminary hack taking what I saw with Alice a bit further towards a self-contained system. My first goal was to get a system where 1) critters dont get smashed in and are freely mobile, 2) the system is stable and doesn’t move around when the foldscope pans from one field to another, 3), the system is sealed to prevent water evaporation.
Here’s what I did :
Materials: 1) Glass slide, 2) thick-PVC (0.016 inches/0.4 mm thick), 3) thin-PVC (0.004 inches/0.1 mm thick, product number 8562K11). See the figure for methods.
Here’s what I did :
Materials: 1) Glass slide, 2) thick-PVC (0.016 inches/0.4 mm thick), 3) thin-PVC (0.004 inches/0.1 mm thick, product number 8562K11). See the figure for methods.

View in Media Gallery
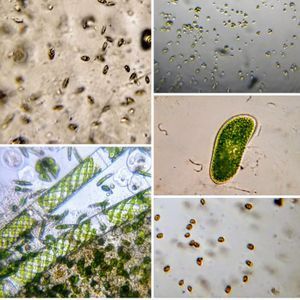
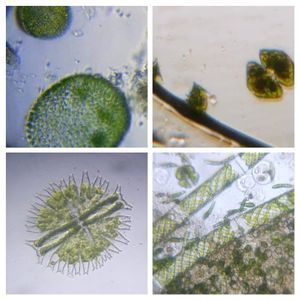
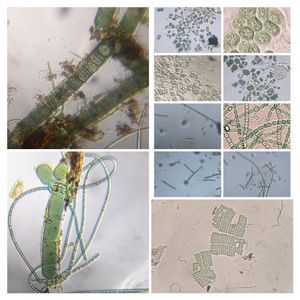
1 . Cut a rectangular ditch in the thick-PVC and place it on a glass slide. Press to get a nice seal. 2. Fill the ditch with the liquid sample (Keep some space, don’t fill it up completely). Put a thin-PVC cover on top and press this along the edge of the ditch so that it seals into the thick-PVC. This is what I would like to term the basic setup. Even this basic setup is very stable: Critters dont get smashed in, liquid samples don’t leak out and the coverslip is very stable. The sample remains in the ditch for as long as you want it. Following are the organisms I saw on the first day from a pond water sample. This is from the same spot I observed last year. My goal is to identify as many organisms as possible from this pond. Note, for unknown organisms, I am using a convention that Manu and I decided upon in an older post ( see comments in https://microcosmos.foldscope.com/?p=16599 for convention).
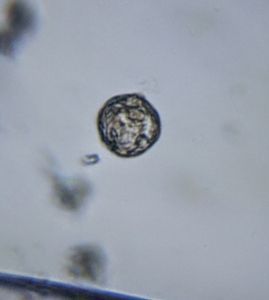
1. Ceratium (SAR->Dinoflagellate). This is my best guess for now, but looks very likely. Looks great in dark field.
1. Ceratium (SAR->Dinoflagellate). This is my best guess for now, but looks very likely. Looks great in dark field.
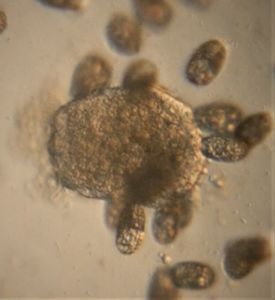
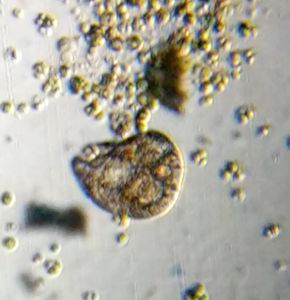
2. Wolfia ; Duckweed (Viridiplantae -> Araceae). Hard to believe that this is an angiosperm, but it does makes a flower and seed.
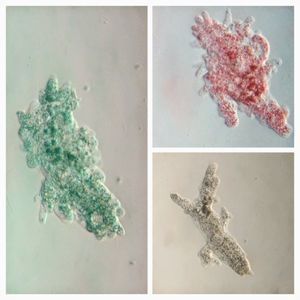
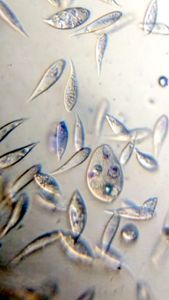
3. Spirogyra (viridiplantae -> Charophyta): Asexual reproduction in these algae is by fragmentation. The fragmentation pathway is clearly seen in the video. I suspect the fragmenting cell apoptoses and the surrounding filaments break off. Note that cell in the middle of the fragmenting alga shows an altered chloroplast morphology.
4. Mougeotia (viridiplantae -> Charophyta): Similar to Spirogyra . Note fragmentation points.Thanks Dr. John Hall (algal expert) for the identification.
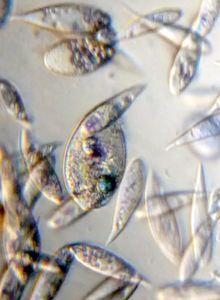
5. Trachelomonas (viridiplantae ->Chlorophyta)
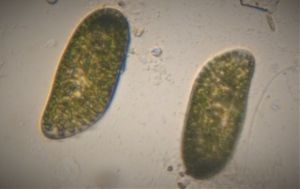
Looks like a moving period. Seen often in freshwater samples. Thanks Dr. John Hall (algal expert) for the identification.
Looks like a moving period. Seen often in freshwater samples. Thanks Dr. John Hall (algal expert) for the identification.
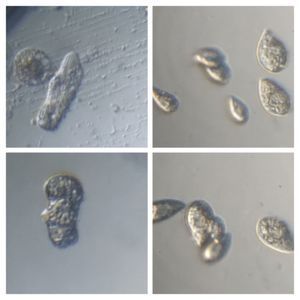
6. Euchlanis-like Rotifer (Metazoa > Rotifera). Rotifer-16915-6-LI
7. Rotifer type II (Metazoa > Rotifera); Rotifer-16915-7-LI. Note the carapace, that looks like cape when it swims about.
8. Cyclops-like Copepod (Metazoa> Crustacea): Note I also observed the nauplis larval form ( see beginning of euplotes video).
9. Flatworm (Platyhelmenthis); Flatworm-16915-9-LI. Stenostomum-like flatworm
10. Unknown Diatom (SAR supergroup > Stramenopiles->Chromista->Bacillariophyta); Diatom-16915-10-LI. They are the little projections lining the charophyte.
11. Euplotes-like spirotrich (Ciliophora); Ciliate-16915-11-LI
Moves like a Euplotes, but not sure if it is the same species. Did not obviously see the cirri, but the contractile vacuoles are really neat.
Moves like a Euplotes, but not sure if it is the same species. Did not obviously see the cirri, but the contractile vacuoles are really neat.
12.Unknown Ciliate; Ciliate-16915-12-LI (Ciliophora)
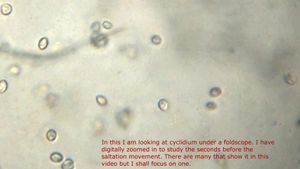
13. Halteria (Ciliophora): Note Saltatory motion of the ciliate.
14. Flagellate (Eukaryota); Flagellate-16915-14-LI. Very fleeting glance.
Now I need to set this up for a few days and see what happens. Smaller volumes makes for a different set of problems. Some more modifications of this basic setup would include sealing this, or perhaps using Agar to make the ditch, which might permit gas exchange. Lots to play with, and I urge you to try this at home. A complete set of the raw videos and pictures can be accessed at https://goo.gl/photos/7mjdsCyhVf2T7LKc9 . I have also uploaded some of these to inaturalist .
Sign in to commentNobody has commented yet... Share your thoughts with the author and start the discussion!

 0 Applause
0 Applause 0 Comments
0 Comments_300x300.jpeg)